DESCRIPTION
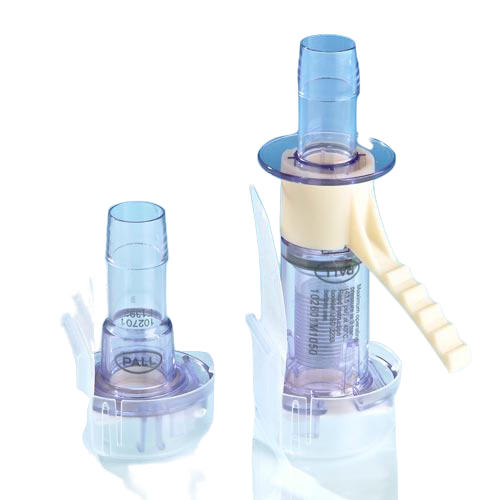
The disposable Kleenpak™ sterile connector, a Pall Life Sciences products, allows for the dry connection of two separate fluid pathways, while maintaining the sterile integrity of both. The connector consists of a male and a female connector, each covered by a vented peel-away strip that protects the port and maintains the sterility of the sterile fluid pathway.
The connector consists of a male and a female connector, each covered by a vented peel-away strip that protects the port and maintains the sterility of the sterile fluid pathway. Different connector options are available to allow for the connection of 15.8 mm (⅝ in.), 13 mm (½ in.), 9.6 mm (⅜ in.) or 6 mm (¼ in.) nominal tubing. Kleenpak sterile connectors can either be autoclaved up to 130°C or Gamma sterilized.
Benefits
No capital equipment required
Connection is made in seconds
Connector can be autoclaved or Gamma sterilized
Easy to make connection – even in cramped environment
Maintains a sterile fluid pathway upon activation of device
Peel-away strip maintains sterility until ready to use
Vented to allow steam penetration and to prevent tubing collapse after autoclaving
Shape coding ensures proper alignment
Locking mechanism prevents incorrect assembly
Device makes audible click when engaged
Permanent connection for security – cannot be re-opened
Quality Standards
Manufactured under clean conditions in a controlled environment
Manufactured under a Quality Management System certified to ISO 9001
Supplied with a Certificate of Test confirming the quality standards and quality control tests performed by Pall (including endotoxin USP and particulate tests)
Validation tests include:
Burst
Creep-rupture
Tensile strength
Aerosol challenge
Shelf-life
Absence of BPA (Bisphenol-A)
Bacterial spore challenge ('soiling') test
Closure integrity
Water flow characteristics
Extractables
Autoclave
The fluid path materials of construction meet:
Biological Reactivity Tests in vivo for Class VI-121°C Plastics, USP
USP Physicochemical Tests for plastics, USP
Connector Validation
Pall offers a comprehensive connector validation service to ensure a smooth product implementation without delay. Validation packages will be tailored to meet your specific needs and will include some or all of the following:
Compatibility assessment
Extractables analysis
Soiling test
Applications
Transfer of inoculums to reactors
Sampling during fermentation or cell culture
Connection of small sterile equipment to large equipment (e.g., bioreactors)
Handling of bulk material in a nonsterile environment
Connection from tank to filling line
Connection to a disposable system
Sensor insertion into bioreactor
Connectors and Disconnectors
 +852-3069 6950
+852-3069 6950 css@gugent.com.hk
css@gugent.com.hk