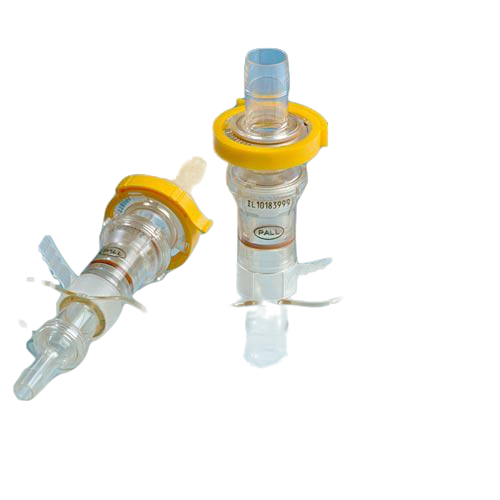
DESCRIPTION
Kleenpak™ sterile disconnectors, a Pall Life Sciences product, provides an easy to use, secure method for the sterile disconnection of flexible tubing assemblies even in an uncontrolled environment.
The disconnectors can also be used in processes involving single-use systems as well as hybrid systems. The disconnector maintains sterility of the fluid path before and after disconnection. The disconnector consists of a joined male and female unit, to fit tubing with 6 mm (1⁄4 in.), 9.5 mm (3⁄8 in.) or 13 mm (1⁄2 in.) ID. Kleenpak sterile disconnectors can either be autoclaved up to 130 °C or gamma irradiated up to 50 kGy.
The Kleenpak sterile disconnector can be used at all stages of the process, but will be particularly valuable in upstream and formulation & filling, where both ease of use and sterility assurance are critical.
Benefits
Disconnection can be performed in non controlled environment without compromising the sterility of fluid path
No capital equipment required
Disconnection is performed in seconds
Easy to make disconnection – even in cramped environments
Microbial control or sterilization by autoclave or gamma irradiation
Activation enables a sterile separation.
Locking mechanism prevents incorrect disassembly
Secure permanent disconnection security – cannot be reconnected
No flow restrictions as a result of using of the Kleenpak sterile disconnector
Warning: This product is not sold sterile. For use in making sterile disconnections, each disconnector must be fitted to a closed single-use assembly that has been subjected to a validated sterilizing process.
Applications
Disconnection after transfer of inocula to fermentors or bioreactors
Disconnection after sampling
Disconnection of small sterile equipment from large equipment (e.g. bioreactors)
Disconnection while handling bulk material in a non sterile environment
Disconnection from tank to filling line
Disconnection from a single-use system
Performance
Quality Standards
Manufactured under clean conditions in a controlled environment
Manufactured under a Quality Management System certified to ISO9001
Supplied with a Certificate of Test confirming the quality standards and quality control tests performed by Pall (including USP and particulate tests)
Validation tests include:
Mechanical tests
Leak; Burst; Creep rupture and pressure hold test
Resistance to freezing storage
Functional tests
Water flow characteristics
Bacterial spore challenge ("soiling") test
Autoclave and gamma resistance
Shelf life studies
Extractables
Absence of BPA (Bisphenol-A) and pthalates
The fluid path materials of construction have been tested and meet the requirements of:
USP Class VI 121°C - Biological reactivity in vivo
USP 87 - Biological reactivity in vitro
USP 661 - Physicochemical tests
The fluid path materials of construction do not contain any substances derived from animal products (i.e. TSE/BSE risk free)
Sterile Disconnections in Less Than 30 Seconds
 +852-3069 6950
+852-3069 6950 css@gugent.com.hk
css@gugent.com.hk